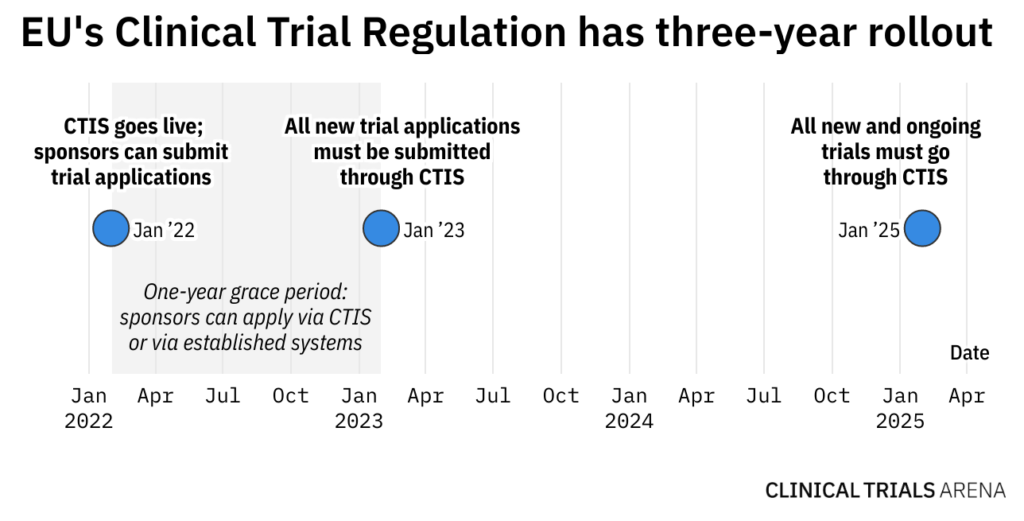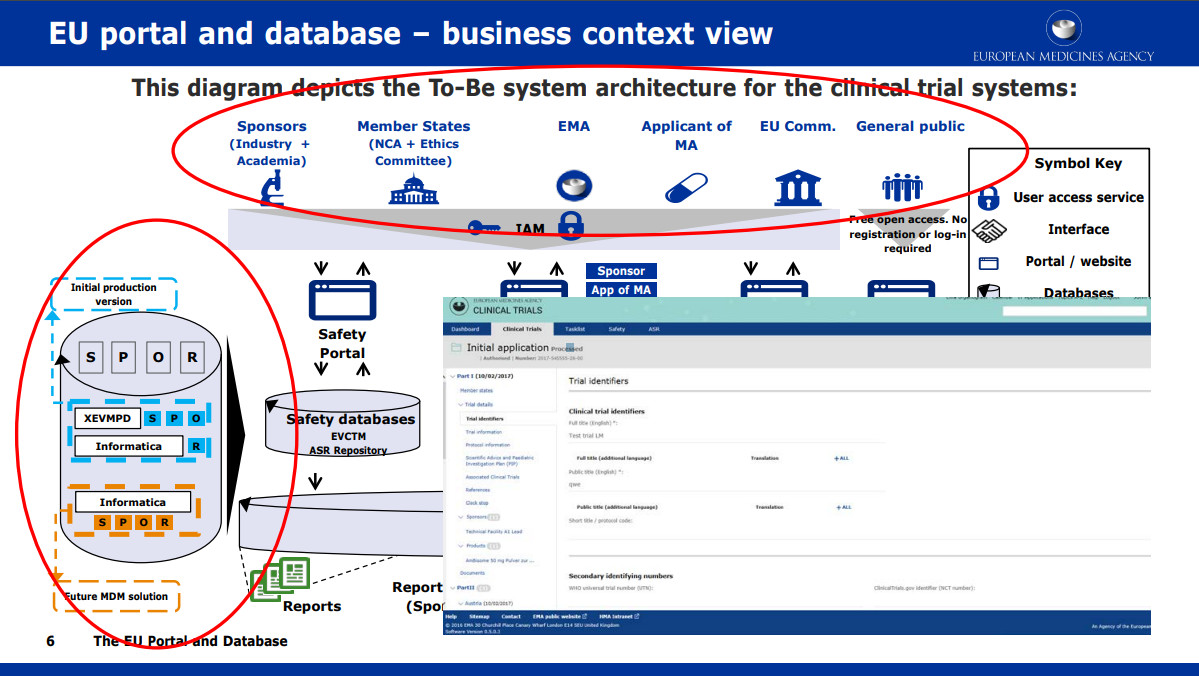
The obligatory sharing of clinical trial data in the European Union - datenschutz notizen | News-Blog der datenschutz nord Gruppe

EU Health - #HealthUnion a Twitteren: "Guidance on clinical trials during the #COVID19 pandemic, including key changes to: ➡️Distribution of the investigational medicinal products ➡️Monitoring ➡️Remote source data verification (SDV ...

Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource | The BMJ

The Current Status of European and National Financial Sources for Clinical Research and Their Impact on Paediatric Non-commercial Clinical Trials: A Case Study of the Czech Republic | SpringerLink

Assessment of the Regulatory Dialogue Between Pharmaceutical Companies and the European Medicines Agency on the Choice of Noninferiority Margins - Clinical Therapeutics

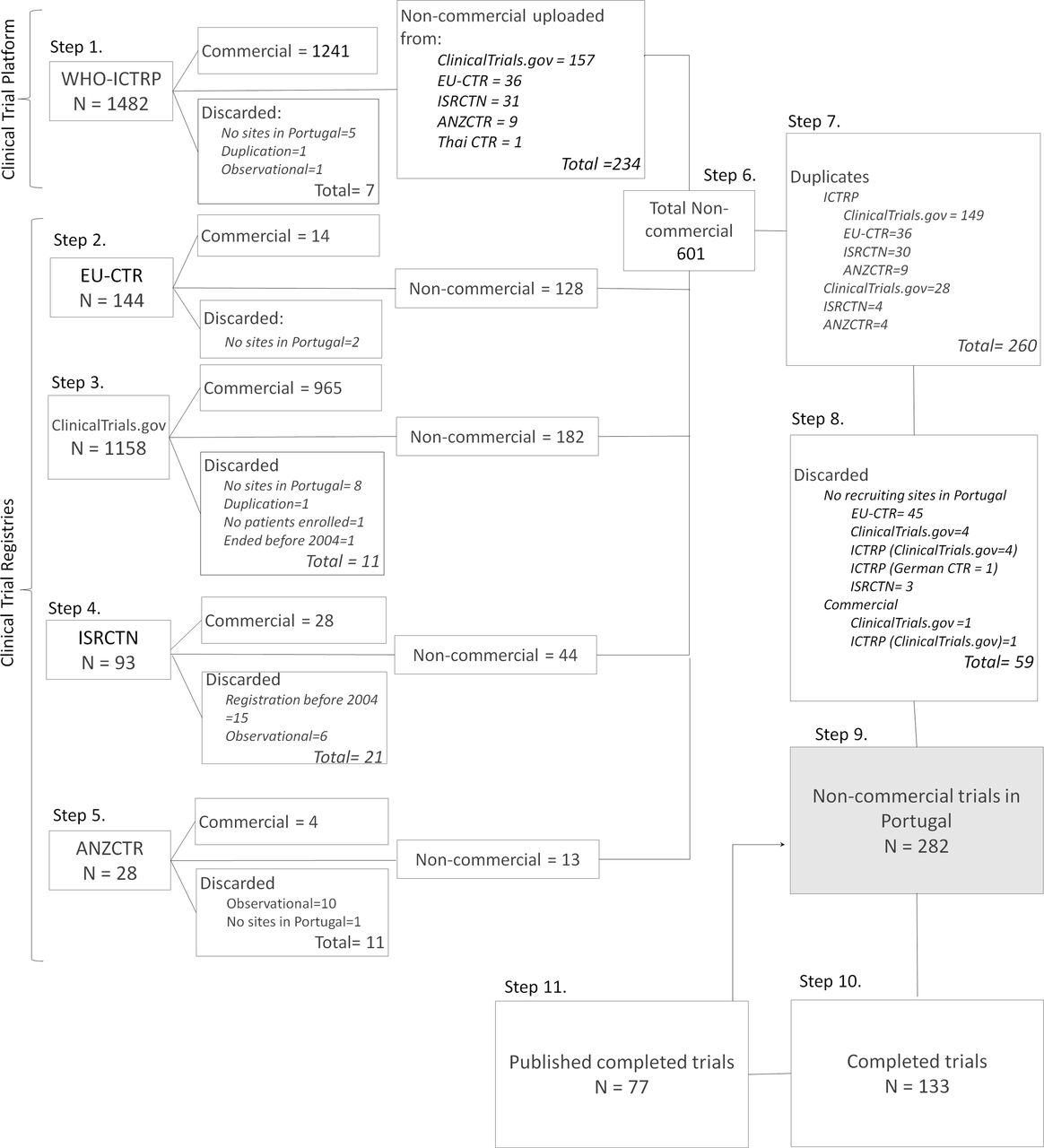
Flow diagram of study design. Abbreviation: EU-CTR, EU Clinical Trials... | Download Scientific Diagram

EU Medicines Agency on Twitter: "In a letter published today, @EU_Commission, #EMA and the Heads of Medicines Agencies remind all sponsors of #ClinicalTrials conducted in the 🇪🇺 to make results of concluded

Flow diagram of the study. Abbreviation: eU-cTr, eU clinical Trials... | Download Scientific Diagram

Cochrane supports European regulators as they urge clinical trial sponsors to share their results | Cochrane
Transparency and accuracy in funding investigator-initiated clinical trials: a systematic search in clinical trials databases | BMJ Open