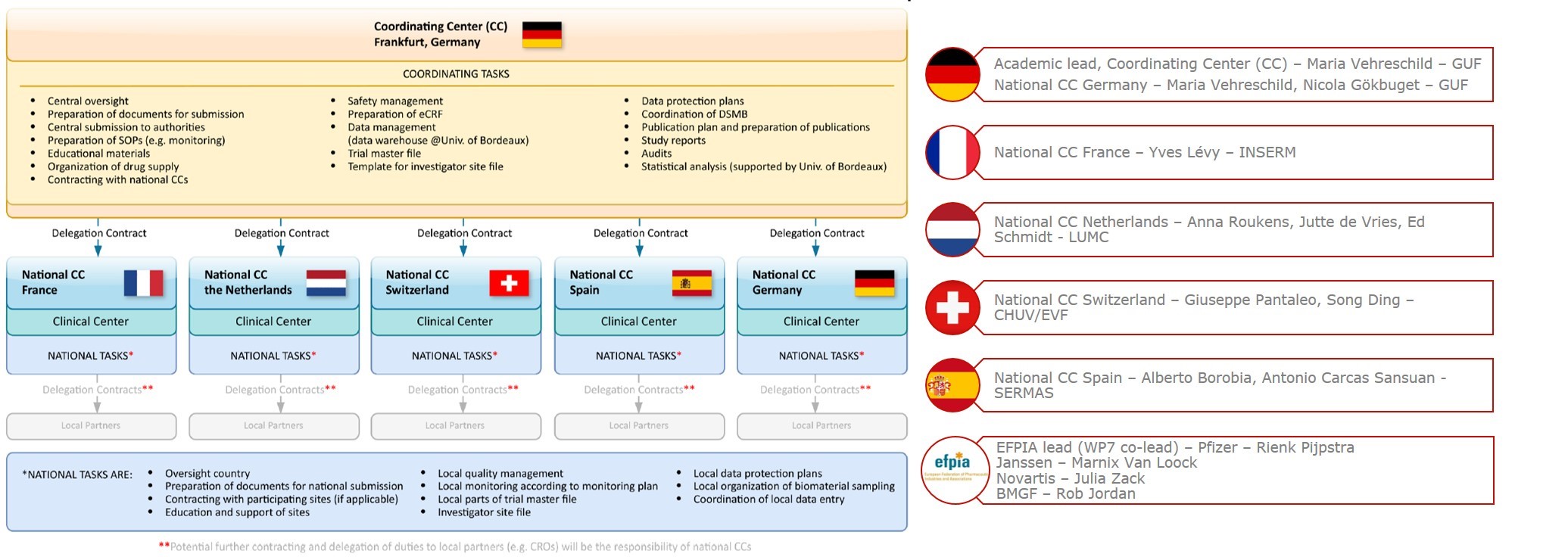
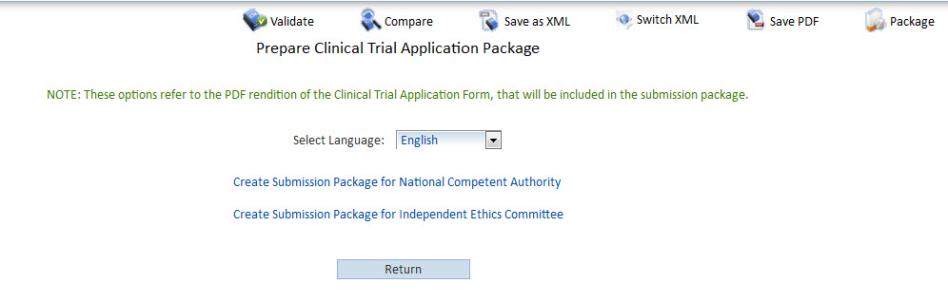
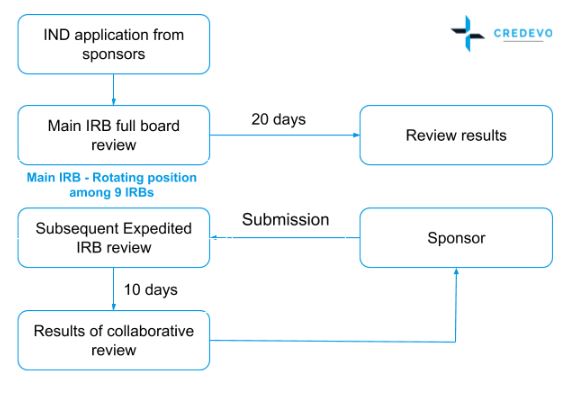
Key steps in the National Cancer Institute (NCI) clinical trial review... | Download Scientific Diagram

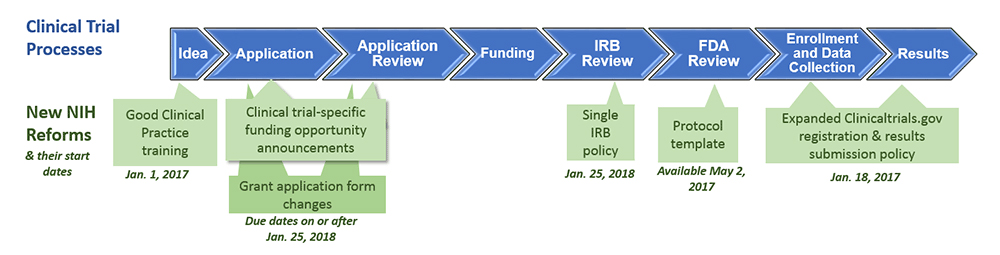
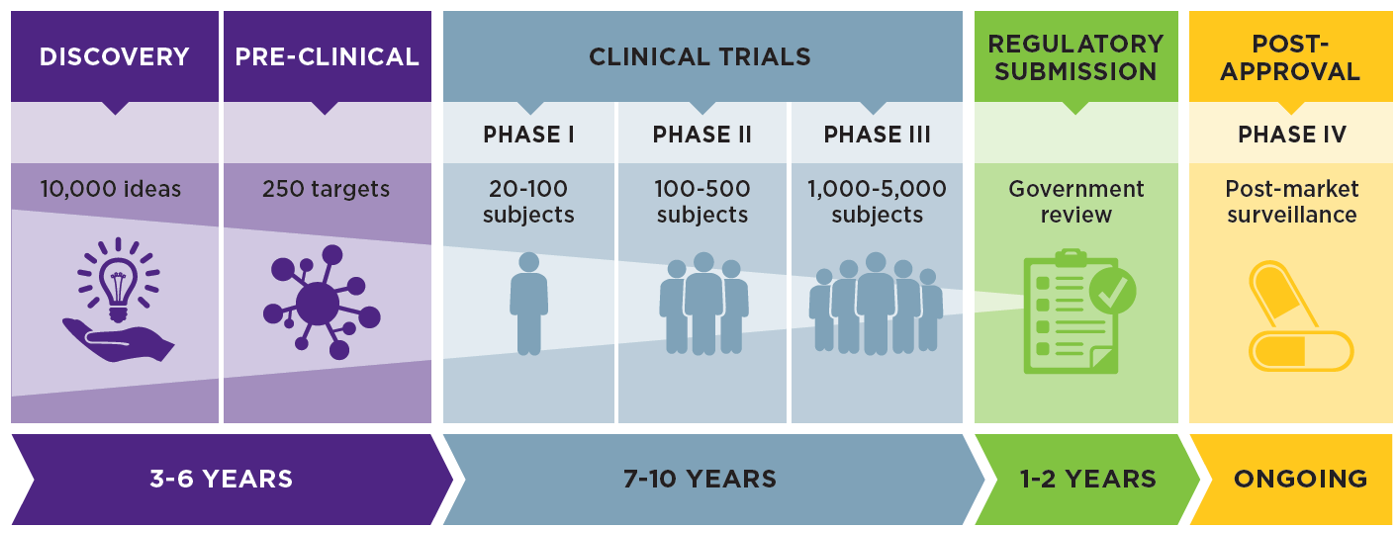
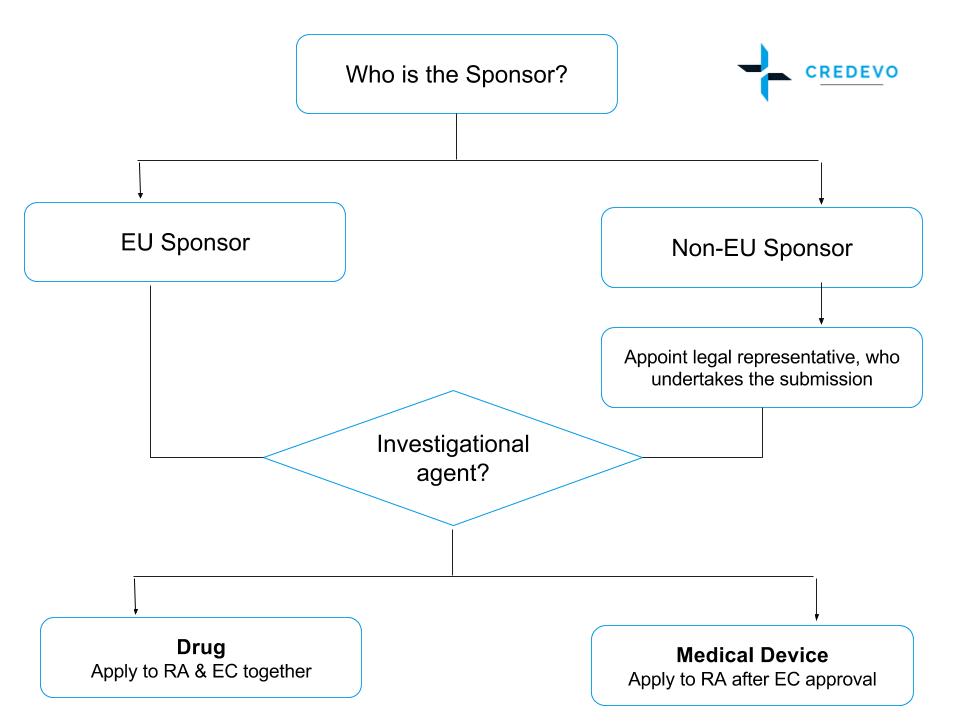
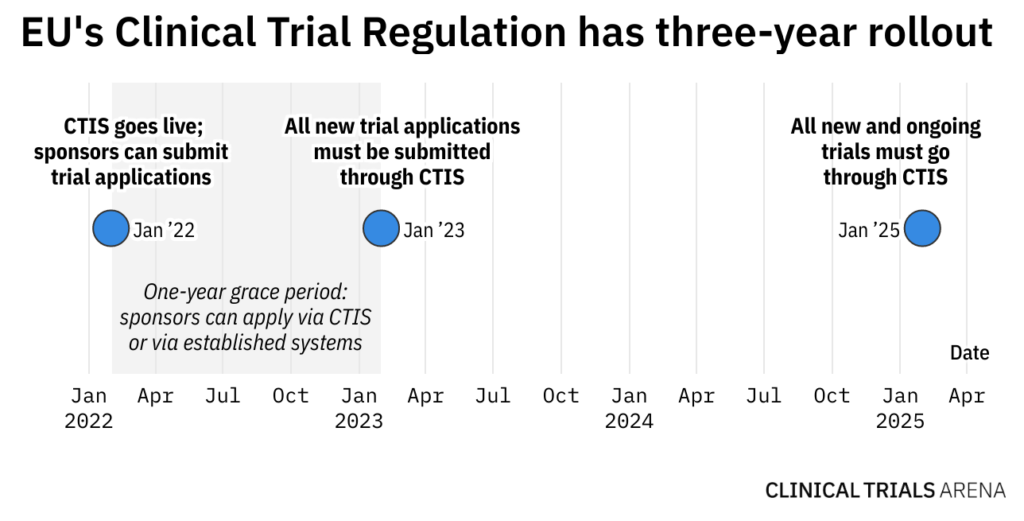
New regulation on clinical trials in Spain - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal
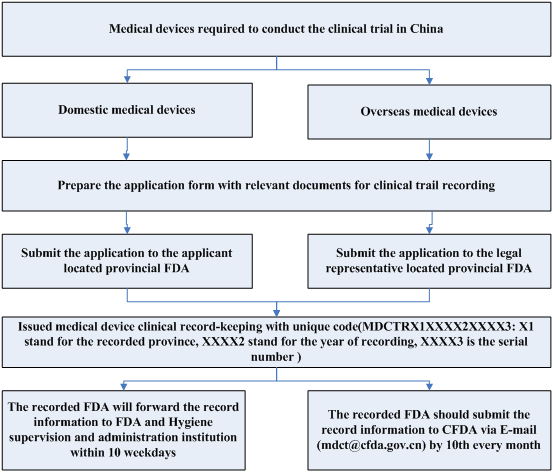
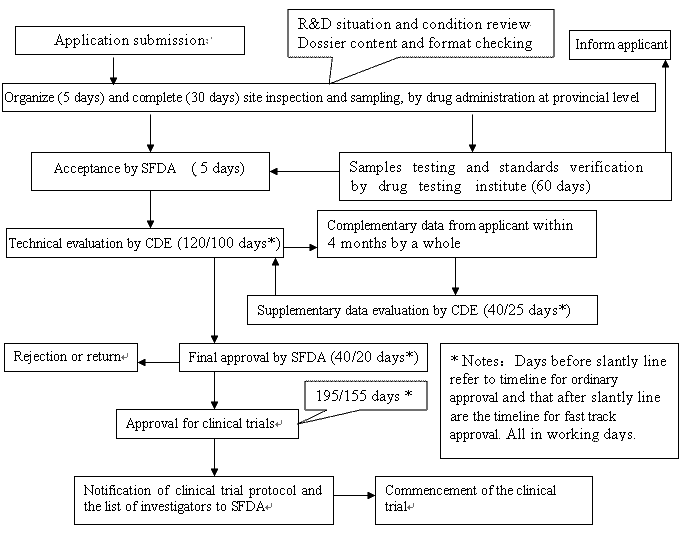
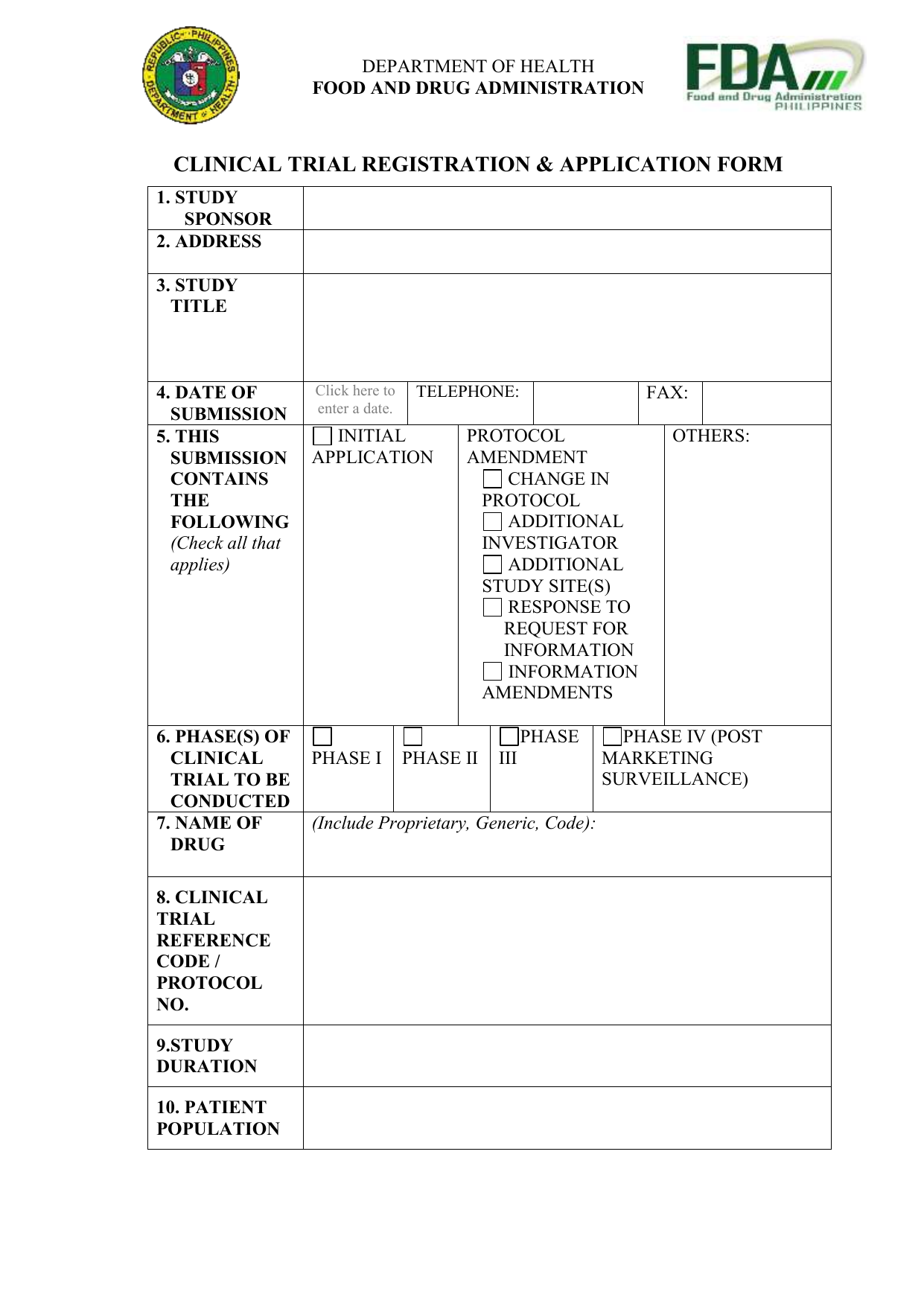
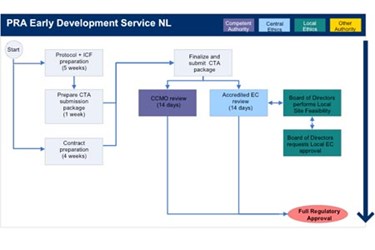
Clinical trial activities required to be recorded in China local FDA - Regulatory News - Medical Devices - CIRS Group

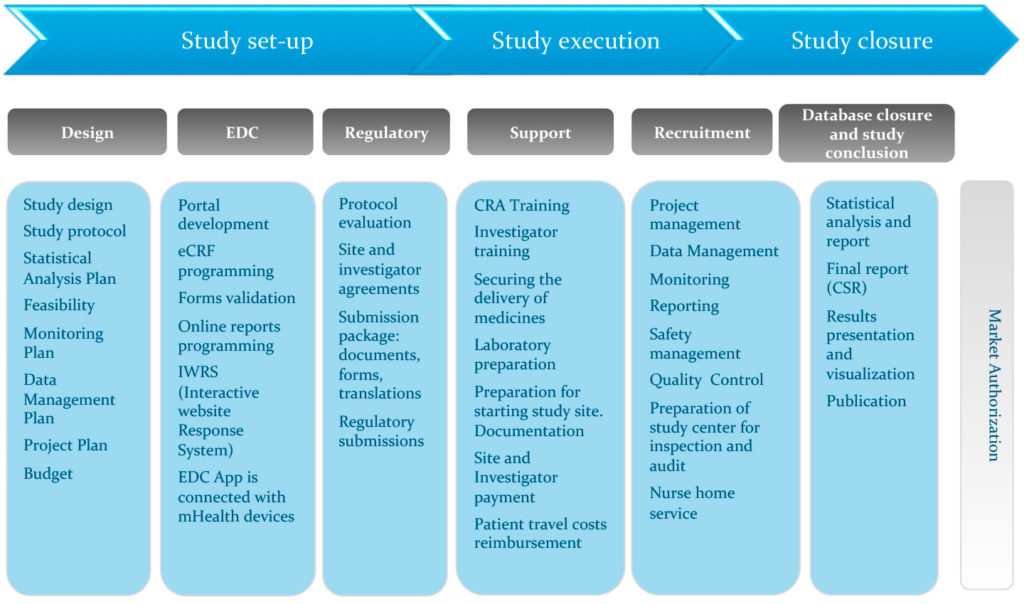
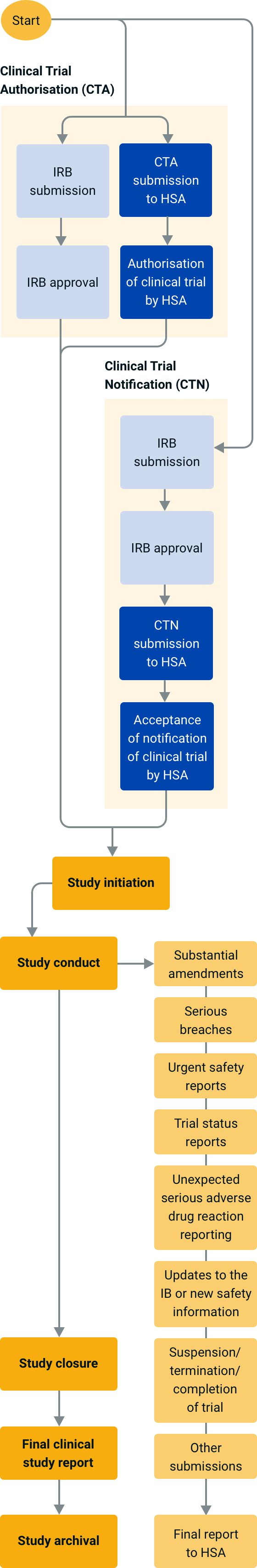
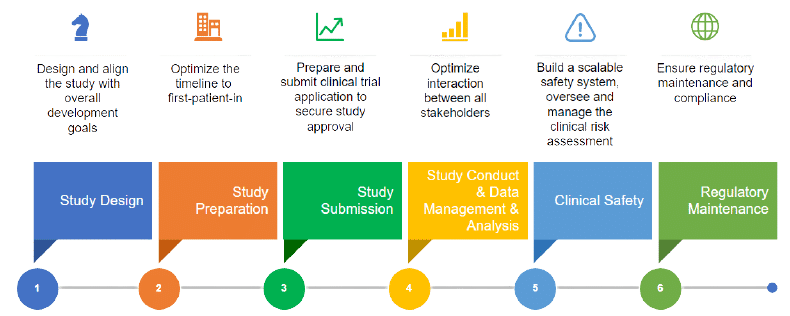
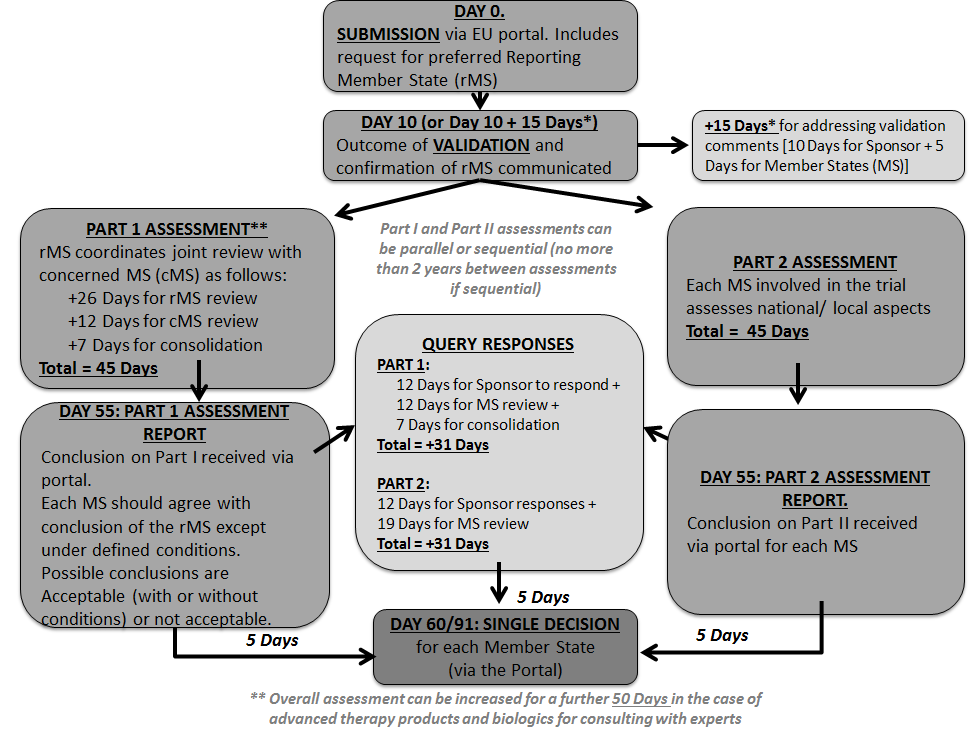
An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram