
What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH
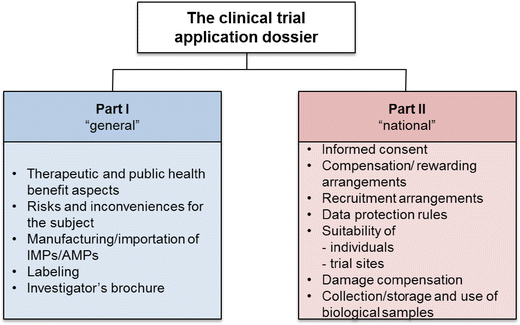
Will the EU Clinical Trials Regulation Support the Innovative Industry in Bringing New Medicines Faster to Patients? | SpringerLink
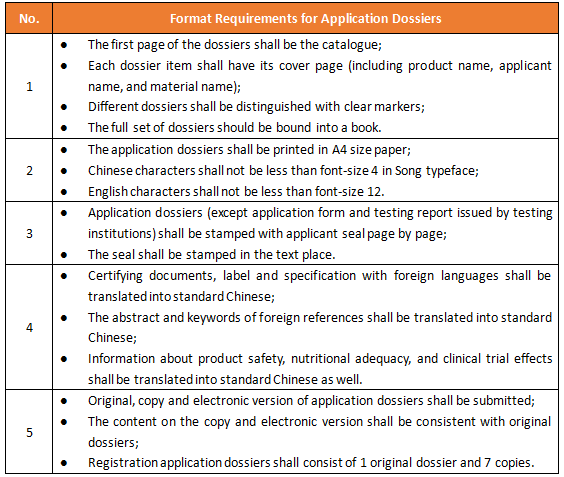
Dossier Requirements of Foods for Special Medical Purpose (FSMP) Registration in China - Regulatory News - Food & Food Contact Materials - CIRS Group

Proposed clinical trial application dossier. AMPs auxiliary medicinal... | Download Scientific Diagram











.png)







